The demand for oligonucleotide production facilities has increased significantly in recent years. As this trend continues, it is essential to design manufacturing facilities that are flexible enough to meet current production needs and have the foresight for future expansion.
An emerging market for patient therapeutics
Given the relative novelty of the process technologies used to manufacture chemically-synthesized oligonucleotides, the current DNA/RNA synthesis methods are primarily carried out at laboratory-scale (batches less than 100 mMol). As large-scale facility designs in the US become more prevalent, scaling up from the pilot lab environment to a full commercial-level facility (>1,000 mMol/day) presents unique infrastructure challenges that have yet to be resolved. When engaging in facility development for oligonucleotides, the outside engineering design and internal client teams should work closely together to understand equipment and material flows, scale limitations, and the intended operational philosophies of each unit operation. For novel therapy facilities, a phased approach is often considered to allow production to begin at an initial, lower throughput while leaving room to grow for future expansion in overall capacity.
Understanding limitations is essential
Arcadis has designed multiple facilities capable of manufacturing oligonucleotides at scales ranging between 150 and 16,000 mMol/day. Designing a facility for any technology is highly dependent on scalability and forecasted market trends, but oligonucleotide facility design is unique in its inherent overlap between an active pharmaceutical ingredient (API) facility (heavy solvent usage) and a biopharmaceutical facility (cleanroom environments), leading to a handful of limitations, including:
High-hazard spaces (H-spaces)
It’s inevitable that the topic of H-spaces arises when looking at a large-scale facility for a process that uses a substantial amount of flammable solvents. A ‘high hazard’ occupancy per the International Building Code (IBC) comes into play when a building generates or processes any material that constitutes a physical health hazard in quantities in excess of those allowed in control areas (also defined by IBC). Typical solvents used in oligonucleotide synthesis, like acetonitrile, toluene, and pyridine, fall under Class 1B solvents per the National Fire Protection Association (NFPA) code, meaning facilities may only store and process a limited amount of solvent before having to be characterized as a high-hazard area. Assuming the facility is designed with an NFPA-approved sprinkler system and utilizes IBC-approved storage containers, the maximum allowable quantity (MAQ) for Class 1B solvents is 480 gallons in storage and 240 gallons to be used in closed processes (less in open processes).
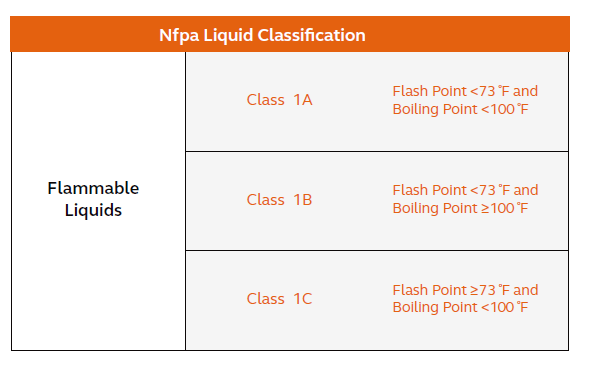
A large-scale facility may have 100 times that in storage and use thousands of gallons of solvent for a single production run, meaning it is unlikely to engineer out the possibility of designing for an H-rated facility.
Designing an H-space is unlike most other bioprocessing facility designs, as code compliance becomes much more rigorous.
For example, H-spaces require:





It is essential to bring an experienced and reliable code consultant on-board early in design, as early as the conceptual phase, to guide code compliance. To confirm all code requirements are sufficiently met, a qualified code consultant will review the production scale, projected chemical inventory and anticipated floor layout and comment on areas that require further evaluation. Ensuring the facility design is fully compliant up-front will remove the need for significant re-work late in the project, adding substantial engineering time and project cost (both of which are at a premium in the era of warp-speed design projects).
Operating at a variety of production scales
For the laboratory environment, equipment selection is fairly trivial; most equipment fits on benchtop spaces, can be moved fairly easily, and doesn’t require an overwhelming capital investment.
At commercial scale, depending on the flexibility required, this is less so. A pilot process producing 100 mMol/day looks quite different than one producing 10,000+ mMol/ day. Synthesis and chromatography equipment, including skids and columns, get larger, taking up valuable space on the production floor. Process skids, supporting feed vessels, distribution equipment, and utility systems must be viable across all production phases. Feed tanks are typically sized for the largest batch they are required to produce, but if they are required to support multiple production scales, they may fall short during turndown. For example, batches less than the minimum required mixing volume of the tank will either need their recipes modified or be mixed in completely separate tanks, which are significant added costs. When considering vessel sizing for both the upper and lower production limits, it will be apparent which proposed pieces of equipment can be repurposed and which will need to be segregated.
-
READ MORE
As equipment sizes increase, the design becomes less mobile. A compromise between dedicated equipment for increased capacity and mobile equipment for flexibility must be met to fulfil the manufacturing needs. Small, portable equipment has a cheaper capital expenditure but requires more manual operation, limiting throughput and repeatability in product quality. Fixed equipment allows for (in many cases) a primarily automated and integrated closed system with little operator intervention but also limits the operable range in production and requires dependable preventative maintenance and cleaning procedures. It is never a one-size-fits-all solution, and this balance can only be achieved with a thorough understanding of the facility’s key process parameters and master operational philosophy.
Other Considerations
There are many other considerations that are no less important than those discussed above to consider in the design phases. A few questions to keep in mind:
Logistics
Aside from production capability, supply chain logistics need to be clearly understood. For a facility potentially using millions of liters of hazardous material per week:



Input from manufacturing
Operability is one of the most critical parameters to evaluate when reviewing a new facility design. Far too often, design projects begin solely with engineering/leadership teams and a goal in mind while not looking at it from a day-to-day perspective. Operations groups (manufacturing, facilities, and maintenance), always bring relevant context to efficiency and safety issues in existing facilities that could easily be mitigated early in the conceptual phases of a new design. Still, this key input is sometimes not highlighted or thought about until design transfers into construction.



Future expansion
Sure, the design may be adequate for current short-term plans, but if the oligonucleotide market rapidly expands in five or ten years, the facility may quickly become obsolete if it is not designed adequately to meet the required capacity. Instead of pursuing an entirely new capital project to catch up, why not build the potential for market expansion in now? Ideally, flexibility is built into the current footprint to allow for future growth, allowing for major early site work to be completed once, while deferring additional process equipment costs and effort until the future demand becomes apparent. “Shell” spaces are unfinished rooms built within the given footprint to be used for an intended purpose later and are a great way to provide flexibility to a facility that may not have the current capacity requirement but is expected to reach market forecasts throughout its lifetime. Consider the following for future design options:



The right solution
The demand for oligonucleotide production facilities has increased significantly in the past year and is likely to continue. Designing a manufacturing facility that is flexible enough to meet the current production need and has the foresight toward future expansion requires partnering with the right A/E/C firm to turn that vision into reality. Whether designing for a pilot- or commercial-scale oligonucleotide facility, Arcadis is committed to developing an engineering solution that works for you.


